Medical and healthcare sector applications
The usage of 3D-printing on medical and healthcare sector applications stems from the beginning of the manufacturing methods.
In recent years, the introduction of the manufacturing methods to known and new applications has sped up as the techniques have developed and become more common. Multiple manufacturers offer wide variety of different machines for health sector needs. It is also noticeable that some manufacturers keep significantly higher price for medical devices, even while the machine is practically the same for other industry use. It should be noted however that material requirements are sometimes stricter for the medical sector than other industries. In addition, the certification processes in health sector for machines, materials and even specific uses can be long and costly.
In United States the usage of 3D-printing in medical and healthcare sector is administered by FDA (Food and Drug Administration). In 2017 FDA published the first guidance to the subject: "Technical Considerations for Additive Manufactured Medical Devices". Source: https://www.fda.gov/medical-devices/products-and-medical-procedures/3d-printing-medical-devices
In Europe 3D-printing usage in the sectors are regulated by MDR (Medical Device Regulation) and IVDR (In vitro diagnostic regulation). Currently there is a transition time as the regulations are being upgraded. The new regulations take effect at:
- 26.5.2020: (2017/745/EU) (MDR)
- 26.5.2022: (2017/746/EU) (IVDR)
Material development has a big role on medical sector applications, for example, traditionally implants have been made of titanium, but in the future, plastics developed specifically for this use will replace some of the applications.
In recent years, the usage of 3D-printing in health sector has decreased compared to other industry sectors. This does not however mean that the usage of 3D-printing on health sector has decreased, but that on other sectors the usage of 3D-printing has grown even faster. Even in Finland, 3D-printing in health care sector has been on the news and on 2013 the first doctoral thesis on the subject was finished. The Finnish hospitals have had considerably low know how of 3D-printing, but in recent years there have been signs that it’s getting better.
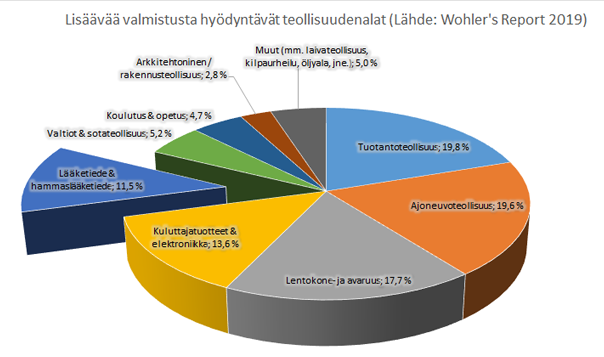
Picture 1. According to Wohler's Report, medical and dentistry sector took up 11.5 % of the use cases of 3D-printing in 2018.
3D bio printing
Bio printing enables printing of living tissue and whole organs. While bio printing is still mostly on research stage, around the world there have been multiple successful cases where 3d-printing has been used to print different organs like ears or trachea.
One of the research subjects of bio printing industry is the printing of blood vessel tissue. Manufacturing blood vessels inside of tissue is technically very challenging process, researchers have been able to do it by first 3D-printing a framework, growing cells around the framework and then dissolving the soluble framework away from the inside of the blood vessel.
In future bio printing has a possibility in addition to repairing ”broken”/”worn” parts to enable “organ upgrades”. On USA, Princeton University project an ear with an integrated radio wave receiver was 3D-printed. This kind of upgrade could make it possible to hear frequencies on a much wider scale than normal human ear is capable of hearing. Even though the subject needs, a lot more research to make it a successful organ upgrade, in principle the integrated received could be connected to human nerve endings and make it work like a hearing aid. The research goal was to be acquainted with electronic and biological structure integration.
Dental care
On dental care sector, there are significant amount of different applications for plastic and metal 3D-printed parts. Some of the applications are operative surgical jigs and models, tools and molds to help traditional techniques and tooth implants, -crowns and -bridges.
On dental care sector 3D-printing markets are estimated to grow 515 %, from current 141 million dollars to 868 million dollars by 2025. Much greater estimates have also been suggested.
Most common use cases on dental care sector are naturally manufacturing of dental implants and alternatives for dental braces.
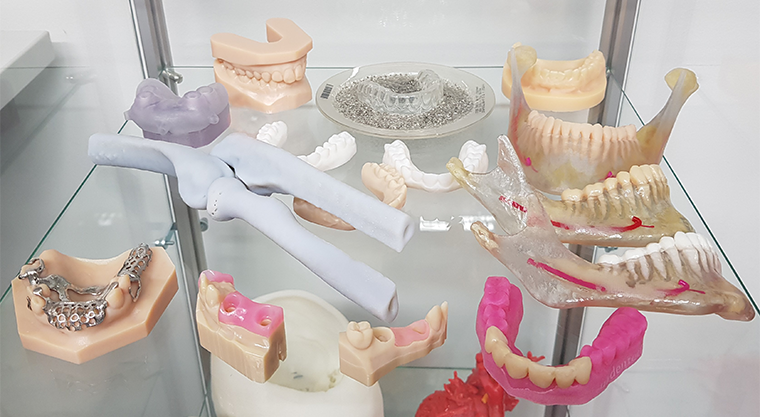
Picture 2. Some of the applications that have sped up the use of 3D-printing on dental care sector. Source: Stratasys 2019
Dental implants, crowns and -bridges
Dentistry applications are probably the most common use case in health care sector. For example Align Technology in United States manufactures daily over 220 000 unique dental braces, which makes the company the biggest actor on this sector in US. (Source: 3dprint.org, 1.6.2018). The intent of the method is to replace traditional dental braces, and the process main steps are approximately:
- The mouth of the customer is 3D-scanned
- An accurete model of the teeth is 3D-printed
- The model is used to manufacture a dental brace from thermoplastics
3D-printing makes the manufacturing process fast and cost effective. Naturally, the company and product has also the approval of medical authorities.
In addition, production of dental implants and crowns with 3D-printing are already commonplace applications. Together with mouth imaging methods, this allows for fast and invidualized solution.
3D-printing continues the development of dental implants: handmade manual work -> CNC-machining -> 3D-printing. 3D-printing has been along CNC-machining for some years as a slightly more costly method. Due to technical development, the method is however replacing CNC-machining as a more cost effective and better solution.
Medicine
With 3D-printing of medicine the goal is for more accurate, patient specific dosages, faster dissolving tablets and to help with the delivery to the patients.
Medicine tablet and dosage 3D-printing
The first prototype printers capable of printing medicine have already been built, and are on use.
In United States the healthcare authories have accepted an 3D-printed tablet “Spritam” for treating epilepcy. The tablet is manufactured with material extrusion like methods. The medicine is spread through nozzle to the printbed and a liquid glue material is sprayed on top of the powder to bind it together. The printbed is a conveyor where the tablets are printed by moving first underneath a powdernozzle, and then from below liquid nozzles. The reason for tablet 3D-printing is that the company manufacturing the Spritam, Aprecia Pharmaceuticals has reckognized problems on tablet dissolving with epilepsy patients. 3D-printing allows the company to control the porosity of the tablets and trough porosity also tablet dissolving rate. According to the manufacturer of Spritam, the tablets dissolve in less than ten seconds. Naturally Aprecia is in the future also going to use the patented manufacturing method ZipDose® for manufacturing other tablets as well.
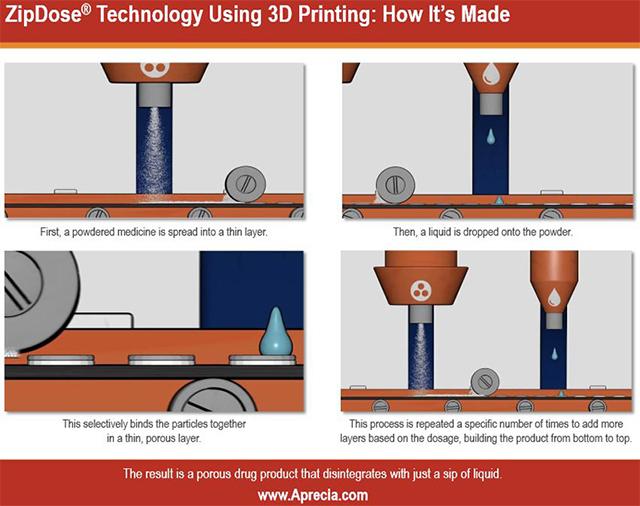
Picture 3. ZipDose method developed by Aprecia, Source: https://www.aprecia.com/zipdose-platform/zipdose-technology.php
There have also been researches for the suitability of other 3D printing methods for producing medicine.
One of the methods that has piqued the interest of researchers is Material extrusion. University College London has researched the use of typical material extrusion method for manufacturing patient tailored tablets. The study was done using commonly available PVA (polyvinyl alcohol) –filament, which was infiltrated with ethanol based fluorescein-solution. The used model drug fluorescein was ethanol-based, because water based medicine-solution would not have worked with water-soluble filament. The actual 3D-printing was done with a common MakerBot Replicator 2X 3D-printer, and the amount of medicine was adjusted using the infill parameter.
According to the research, the method could work for manufacturing patient tailored tablets. The same research group has also been acquainted with possibilities and meaning of different geometries for the tablets.
Solutions for using liquid materials are also researched. One solution could be a medical ink. The materials would be made into liquid form, which could then be printed as tablets. The aim in this concept would be that patients could print patient specific dosages either at the drug store, or buy the raw materials and print the medicine at home, or at a nearby printing service.
Even if the actual medicine were to be manufactured closer to the customer, according to specialists, the manufacturing of raw materials would stay on the hands of big medical companies, because of cost and resource reasons. Only big medical companies have enough resources to develop new medicines and acquire the approval of medical authorities in different countries. This and the legislations are the biggest questions concerning the 3D-printing of medicines. If the medicine is produced by someone else than the medical companies, who is responsible about that the medicine is what it is supposed to be? And what about if the medicine is produced by the pacient? Does 3D-printing make it easier to copy and forge medicines? Will the 3D-printers make it possible to manufacture illegal medicines or drugs?
Sources and additional information
- https://www.aprecia.com/zipdose-platform/zipdose-technology.php
- Mustafa Alomari, Fatima H. Mohamed, Abdul Basit, Simon Gaisford, Personalised dosing: “Printing a dose of one’s own medicine”, University College London, International Journal of Pharmaceutics 494 (2015) s.568–577
- Alvaro Goyanes, Asma B.M. Buanz, Abdul W. Basit, Simon Gaisford: "Fused-filament 3D printing (3DP) for fabrication of tablets", University College London, International Journal of Pharmaceutics 476 (2014) s.88-92
- Shaban A. Khaleda, Jonathan C. Burleya, Morgan R. Alexandera, Jing Yangb, Clive J. Robertsa: "3D printing of tablets containing multiple drugs with defined release profiles", The University of Nottingham, International Journal of Pharmaceutics 494 (2015) p.643-650
- Alvaro Goyanes, Pamela Robles Martinez, Asma Buanz, Abdul W. Basit, Simon Gaisford: “Effect of geometry on drug release from 3D printed tablets”, University College London, International Journal of Pharmaceutics 484 (2015) s.657-663
Devices and parts
Devices for consumers
The medical care device parts aimed for consumer use have so far been restricted by strict approval procedures.
Picture 4. Syqe Inhaler -inhalator device, source: Syqe medical, http://www.syqemedical.com/
Research devices
Common parts for research devices are for example different flanges and adapters.
Lately there have been breakthroughs in printer speeds and quality, and in the usable materials.
Savonia is collaborating with Kuopio University Hospital imaging center - amoung other things there has been research cases on different hearth phantom- devices for radioactive liquid imaging.
Anatomically correct models
Anatomically correct 3D-models have been manufactured for illustrating purposes from the beginning of 3D printing. Through the advancements, the quality of the printed models has improved significantly. In addition, the manufacturing process (imaging/scanning, printing) has become faster and easier, and has obtained a better quality-price-ratio. Manufacturing external limbs and features have become especially easy trough advances and generalization of 3D scanning. Internal organs are usually manufactured using CT scanning to get the accurate patient specific 3D-models.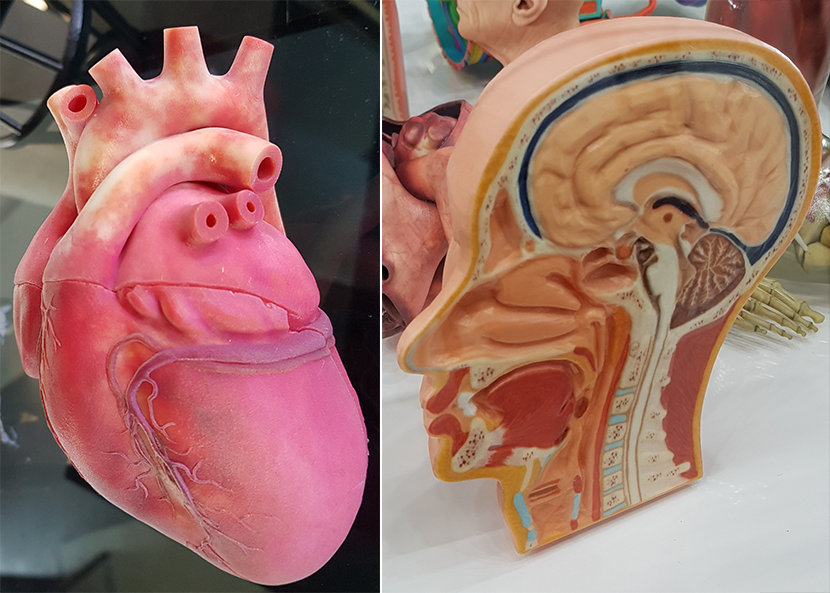
Picture 5. The use of 3D-printed anatomical models for planning surgical operations and teaching is a fast developing trend in the world. Source: Stratasys 2019 & Formnext 2018
Casts/replacing support structures
3D printing allows for patient tailored casts and supports for multiple different uses. In addition to perfect fit, this allows for visually pleasing implementations and multipurpose support structures. 3D printing can also be used for different integrations with electronic devices; one of the examples below is a cast for a foot, with integrated Bluetooth speaker.Prosthesis
3D printing of prosthesis is a fast growing industry. 3D printing allows the manufacturing of complex and nested structures at the same time without additional costs, which makes the process especially suitable for this purpose.
In addition, 3D printing allows the manufacturing of significantly cheaper prosthesis. There are multiple non-profit, charity based multinational projects, which aim to produce cheap prosthesis among others, for developing countries. 3D printing allows for fast tailoring and cheap manufacturing costs.
The cost of a modern versatile prosthesis is easily tens of thousands of euros, while using 3D printing a functioning prosthesis with the cost of couple of hundred euros is a possibility. Naturally, the properties of a cheap prosthesis are not as good as with a top of the line prosthesis, but the quality-price ratio is significantly better. Some studies even point out that many end users rather select a better tailored, otherwise more restricted model than an expensive top of the line prosthesis.

Picture 8. A child’s 3D printed hand prosthesis, Source: http://enablingthefuture.org, 17.8.2015

Picture 9. Exiii-hackberry 3D printable, open-source hand prosthesis, Source: http://exiii-hackberry.com/, 23.8.2019

Picture 10. Patient tailored prosthesis are becoming more common around the world. Source: Formnext 2017
Implants
Like Prosthesis, Implants are one rapidly growing 3D printing area. Implants can be categorized by the material to two categories, soft implants (eg. silicone) and hard implants (eg. titanium). Implants could also be categorized by the stress/strain of the use case, for example hard implants are usually for use cases where the material has to be able to handle stress like bone, joint, etc. while soft implants are more for cosmetic or for low stress use cases.Soft implants
Silicone as a 3D printable material has made it possible to print soft tissue implants. In addition to silicone, an example of soft implants could be for example bio printed hearth aortic valve, which is under research at Cornell University in United States.
Hard implants
Hard implants are the most traditional and common use for implant 3D printing. The used material is often titanium or some plastics. The manufacturing method makes it possible to manufacture perfectly fitting and invidualised implants from CT-scan data. 3D printing also helps to eliminate the problems of poorly fitting implants and the need for manual customization work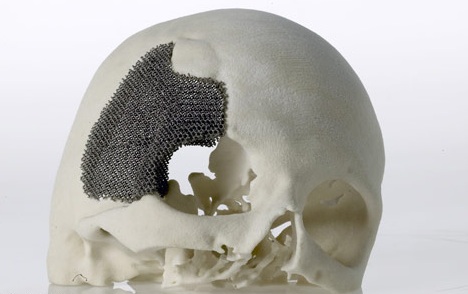
Picture 11: Titanium skull implant for an Argentinian patient, (Source: Arcam & http://www.3dnatives.com/implant-cranien-imprime-3d/ 17.6.2014)
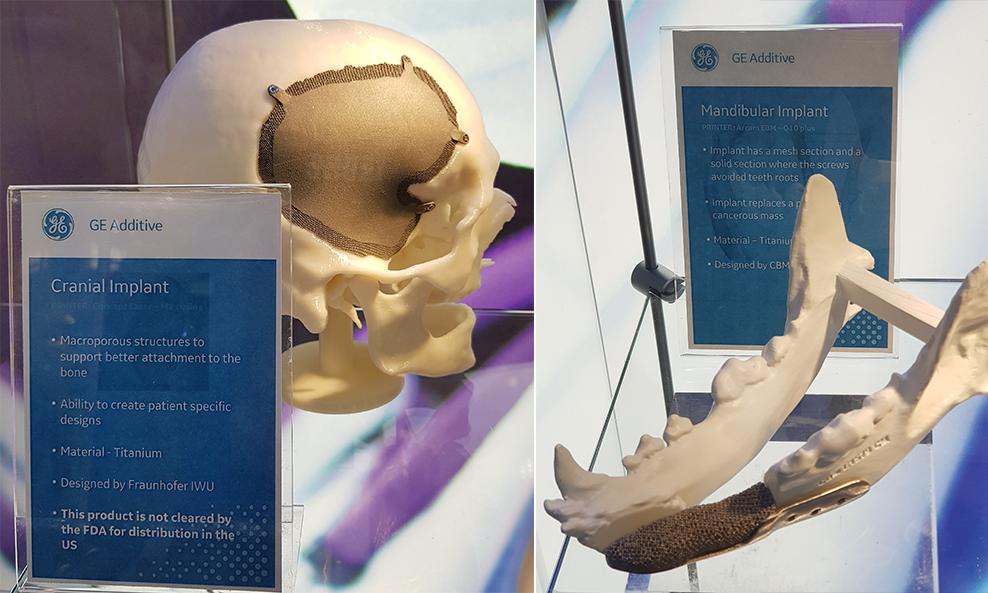
Picture 12. Implant manufacturing for the use of medical sector is becoming more common around the world. Differences on approval processes between countries. Source: GE/Formnext 2018

